Learn how to measure the specific gravity of a battery using a hydrometer to assess its state of charge and health. Step-by-step guide, tips, and correction factors included.
What is Specific Gravity?
Specific gravity is the ratio of the weight of a liquid to the weight of an equal volume of water. It is used to compare the density of different liquids relative to water.
Definition of Specific Gravity in a Battery
Specific gravity in a battery refers to the ratio of the electrolyte’s weight to the weight of an equal volume of pure water. It is used to measure the concentration of sulfuric acid in the electrolyte, indicating the battery’s charge level.
Pure water has a specific gravity of 1.000.
Lead-acid batteries use an electrolyte containing sulfuric acid. Since pure sulfuric acid is 1.835 times heavier than pure water per unit volume, its specific gravity is 1.835.
Because the battery electrolyte is a mixture of water and sulfuric acid, its specific gravity ranges between 1.000 and 1.835. Typically, battery electrolytes are prepared with a specific gravity of less than 1.350 to ensure optimal performance.
What is Battery Acid?
Battery acid refers to the electrolyte found in batteries, primarily sulfuric acid (H₂SO₄) in lead-acid batteries. It is a strong, colorless, and odorless acid that plays a crucial role in the electrochemical reactions within the battery.
Importance of Measuring the Specific Gravity and Density of Battery Acid
Measuring the specific gravity of the battery electrolyte helps estimate the state of charge. During discharge, chemical reactions cause the sulfuric acid concentration to decrease, lowering the electrolyte’s specific gravity.
By checking the density of battery acid, the H₂SO₄ concentration and battery charge status can be determined.
The results help operators decide whether the battery requires minor maintenance or a complete replacement.
Regular density checks are essential for identifying weak cells and ensuring efficient battery performance.
How to Measure Specific Gravity of Battery
The specific gravity of the battery electrolyte can be measured using:
- Hydrometer (Aerometer): A manual device that measures the density of the electrolyte.
- Digital Density Meter (Digital Hydrometer): An electronic instrument that provides accurate and quick readings of the electrolyte’s specific gravity.
Measuring Specific Gravity with a Hydrometer
A hydrometer (aerometer) is a device used to measure the specific gravity of liquids, including battery electrolytes. In battery testing, it helps determine the sulfuric acid concentration in the electrolyte, which directly indicates the battery’s state of charge—a higher specific gravity means a higher acid content.
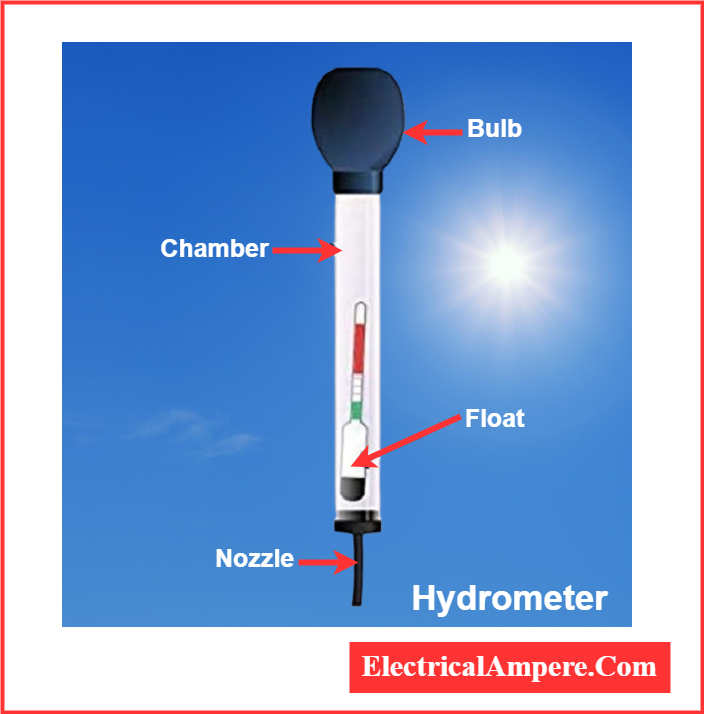
The IEEE recommends recording hydrometer readings over time as part of a Battery Maintenance Program to monitor battery health and performance.
A lead-acid battery hydrometer is a specialized instrument that resembles a syringe with a bulb and a calibrated float inside. To use it, a small amount of battery acid (H₂SO₄) is drawn into the hydrometer bulb. As the float inside the bulb settles, the specific gravity value can be read directly from the float scale. This reading helps determine whether the battery is fully charged, partially charged, or in need of maintenance. Regular monitoring of specific gravity ensures better battery performance and longevity.
Measuring Specific Gravity with a Digital Density Meter
A digital density meter (also known as a digital hydrometer) is an advanced tool for measuring the specific gravity of the sulfuric acid electrolyte in batteries. As long as the measuring cell is resistant to aggressive acids, it can accurately determine the specific gravity.

The meter automatically adjusts for temperature variations and displays the result digitally in the appropriate unit, such as SG (Specific Gravity) 80/80.
Compared to traditional hydrometers, digital density meters offer several advantages. They are easier and faster to clean while providing instant, temperature-compensated readings on a digital screen. Since sample sizes remain consistent, rinsing between measurements is sufficient, eliminating the need for extensive cleaning.
Additionally, a small sample volume of just 2 mL is required for accurate measurements. By following a few simple guidelines, it is possible to obtain precise density readings, making digital density meters a highly efficient tool for battery maintenance.
Using the specific gravity values in the table below, one can determine the depth of discharge (DOD) of the battery cell from which the electrolyte sample was taken.
| DOD (Depth of Discharge) | 2V Battery | 12V Battery | 24V Battery | 48V Battery | Specific Gravity |
|---|---|---|---|---|---|
| 0% | 2.10 | 12.70 | 25.40 | 50.80 | 1.26 |
| 10% | 2.09 | 12.58 | 25.16 | 50.32 | 1.25 |
| 20% | 2.08 | 12.46 | 24.92 | 49.84 | 1.23 |
| 30% | 2.06 | 12.36 | 24.72 | 49.44 | 1.22 |
| 40% | 2.05 | 12.28 | 24.56 | 49.12 | 1.20 |
| 50% | 2.03 | 12.20 | 24.40 | 48.80 | 1.19 |
| 60% | 2.02 | 12.12 | 24.24 | 48.48 | 1.17 |
| Fully Discharged | 1.75 | 11.90 | 23.80 | 47.60 | 1.12 |
Temperature Correction for the Specific Gravity of Acid
The specific gravity (SG) of battery acid varies with temperature. In extreme heat or cold, readings may become inaccurate. To correct for temperature variations, use the following formulae or adjust the SG by 0.003 points for every 10°F deviation from 70°F.
Correction Formulas:
- For Fahrenheit:
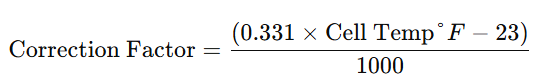
(Equivalent to ±0.003 points per 10°F)
- For Celsius:

These corrections are accurate for temperatures ranging from -17.8°C to 54.4°C (0°F to 130°F).
Open-Circuit Voltage Test
To obtain accurate voltage readings, batteries must remain idle (neither charging nor discharging) for at least six hours, and preferably twenty-four hours before testing.
Steps for Testing:
- Disconnect all loads from the batteries.
- Use a DC voltmeter to measure the battery’s voltage accurately.
- Refer to the table below to estimate the battery’s state of charge.
- Charge the battery if its charge level is between 0% and 70%.
- If the voltage reading is lower than the table values, one of the following issues may be present:
- The battery remained in a discharged state for too long.
- A battery cell is defective.
In such cases, the battery should either be tested by a specialist or removed from service.
The table below shows the relationship between state of charge, specific gravity, and open-circuit voltage.
| State of Charge (%) | 12V Battery (V) | 24V Battery (V) | 48V Battery (V) |
|---|---|---|---|
| 100% | 12.73 | 25.46 | 50.93 |
| 90% | 12.62 | 25.24 | 50.47 |
| 80% | 12.50 | 25.00 | 49.99 |
| 70% | 12.37 | 24.74 | 49.49 |
| 60% | 12.24 | 24.48 | 48.96 |
| 50% | 12.10 | 24.20 | 48.41 |
| 40% | 11.96 | 23.92 | 47.83 |
| 30% | 11.81 | 23.63 | 47.26 |
| 20% | 11.66 | 23.32 | 46.63 |
| 10% | 11.51 | 23.02 | 46.03 |
Conclusion
Understanding how to measure the specific gravity of a battery is essential for maintaining its performance and longevity. By using a hydrometer and applying temperature corrections, you can accurately assess the battery’s state of charge and overall health. Regular monitoring helps prevent premature failure and ensures optimal operation. Follow the proper testing procedures and safety precautions to keep your battery in top condition.
Related Articles:
- Factors Affecting Lead Acid Battery Life
- Battery Acid: Name, pH, and the Acid Used in Car Batteries Explained
- Difference Between AA and AAA Battery
- Buckling of Lead Acid Battery – Causes, Effects & Prevention
- Lead Acid Battery: Construction, Working, Diagram & Reactions
- What is a Sulfated Battery and How to Prevent It
- Rechargeable and Non-Rechargeable Batteries