Batteries are part of our everyday life, from powering cars to storing backup energy for homes and industries. When people talk about a battery with acid, they are usually referring to the most common type of rechargeable battery: the lead-acid battery. At the core of this battery is a special liquid known as battery acid, which acts as the electrolyte that allows the battery to store and release electrical energy.
What is Battery Acid?
Battery acid is the liquid electrolyte inside a lead-acid battery. This liquid is a mixture of sulfuric acid (H₂SO₄) and water. It plays a vital role in storing and releasing electrical energy through chemical reactions between the electrolyte and the lead plates inside the battery.
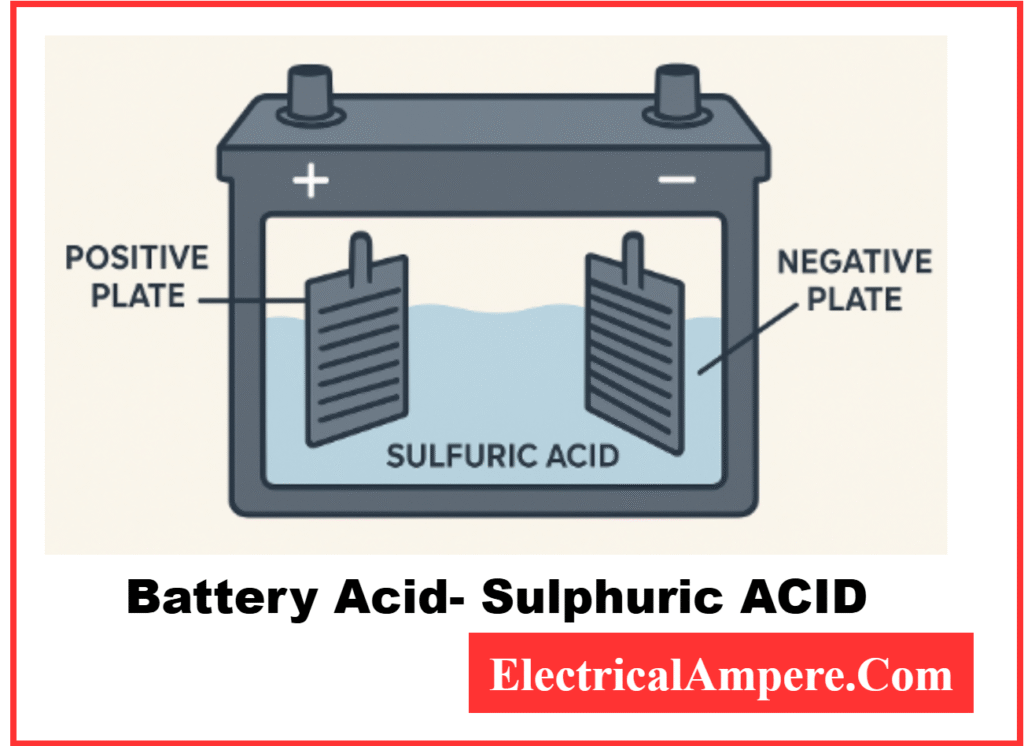
Without this electrolyte, a battery would not be able to function. The acid allows ions to flow between the battery’s positive and negative plates, which generates electricity.
Battery Acid Name: What is it Called?
The chemical name of battery acid is sulfuric acid, a strong and highly corrosive mineral acid. In chemistry, its sulfuric acid chemical name is H₂SO₄. In its pure form, sulfuric acid is a dense, colorless, oily liquid. Inside a battery with acid, it is diluted with water to achieve the proper concentration for safe and effective operation.
Sulfuric Acid Names and Concentrations
Sulfuric acid is known by different names depending on its concentration and usage:
| Sulfuric Acid Name | Concentration | Notes / Usage |
| Dilute sulfuric acid | <29% or 4.2 mol/L | Commonly used in labs and industrial processes |
| Battery acid | 29–32% or 4.2–5.0 mol/L | Typical concentration in lead-acid batteries |
| Chamber acid / Fertilizer acid | 62–70% or 9.2–11.5 mol/L | Made using the lead chamber process |
| Tower acid / Glover acid | 78–80% or 13.5–14.0 mol/L | Recovered from the bottom of the Glover tower |
| 66 °Bé acid | 93.2% or 17.4 mol/L | Named after density measured using a hydrometer |
| Concentrated sulfuric acid | 98.3% or 18.4 mol/L | Nearly 100%, loses SO₃ near boiling point |
When referring to a battery with acid, we are usually talking about sulfuric acid in the 29–32% concentration range, which is ideal for lead-acid battery performance.
Which Acid is Used in Car Batteries?
One of the most common queries is: which acid is used in car batteries?
The answer is sulfuric acid. Car batteries are lead-acid batteries that use sulfuric acid mixed with distilled water. The typical concentration of sulfuric acid in a car battery ranges between 30–50%, depending on whether the battery is fully charged or discharged.
When the battery discharges, sulfuric acid reacts with the lead plates to form lead sulfate, releasing electrical energy that powers the car. When recharged, the process reverses, restoring sulfuric acid concentration.
Battery Acid pH: How Strong is it?
The pH of battery acid is usually between 0.8 and 1.0, which makes it extremely acidic and corrosive. To put this in perspective:
- Water (neutral): pH 7
- Vinegar: pH 2–3
- Acid: pH less than 1
This shows why even a small amount of acid can cause severe burns to skin or damage materials like clothes and metal.
Properties of Battery Acid (Sulfuric Acid)
To understand how battery liquid works, let’s look at some of its important properties:
- Appearance: Clear, colorless liquid (though inside a battery it may appear slightly cloudy).
- Density: Around 1.25–1.30 g/cm³ depending on charge level.
- Corrosiveness: Highly corrosive to skin, eyes, and metals.
- Conductivity: Excellent conductor of electricity when dissolved in water.
- Chemical formula: H₂SO₄ (sulfuric acid chemical name).
Role of Battery Acid in a Lead-Acid Battery
The battery liquid is more than just a filler. It is the heart of the chemical process:
- During discharge: Sulfuric acid reacts with the lead dioxide (positive plate) and lead (negative plate) to form lead sulfate and water. This releases electricity.
- During charging: The reaction reverses. Lead sulfate converts back into lead dioxide, lead, and sulfuric acid, restoring the electrolyte concentration.
This constant cycle makes sulfuric acid one of the most important industrial chemicals in the world.
Construction and Chemical Reactions of a Lead-Acid Battery
A lead-acid battery, the most common type of rechargeable battery, is made up of two lead-based plates—one positive and one negative—immersed in a liquid of sulfuric acid diluted with water. This electrolyte enables the battery to store and release energy through chemical reactions.
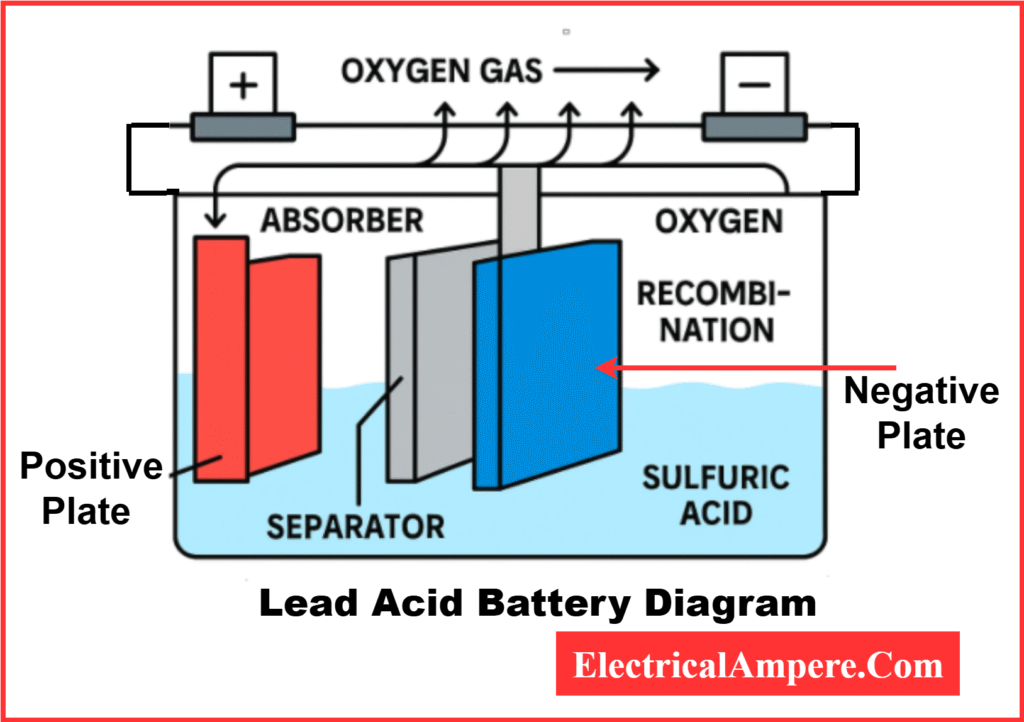
When the battery is in use (discharging), electrons flow from the negative lead plate to the positive plate, producing electrical energy. The chemical reactions at each plate drive this flow:
At the negative plate (anode):
Pb(s) + HSO₄⁻(aq) → PbSO₄(s) + H⁺(aq) + 2 e⁻
At the positive plate (cathode):
PbO₂(s) + HSO₄⁻(aq) + 3 H⁺(aq) + 2 e⁻ → PbSO₄(s) + 2 H₂O(l)
The overall reaction during battery discharge can be written as:
Pb(s) + PbO₂(s) + 2 H₂SO₄(aq) → 2 PbSO₄(s) + 2 H₂O(l)
Charging and Discharging Process
When the battery is fully charged, the negative plate consists of lead (Pb), the positive plate is lead dioxide (PbO₂). And, the electrolyte contains a higher concentration of sulfuric acid. During discharge, these materials react with the acid to generate electricity, forming lead sulfate (PbSO₄) on both plates and diluting the acid.
If the battery is overcharged, water in the electrolyte undergoes electrolysis, releasing hydrogen and oxygen gases, which can escape from the battery. Some lead-acid batteries are designed so you can top up with distilled water to maintain proper electrolyte levels.
After a full discharge, both plates are coated with lead sulfate, and the electrolyte becomes mostly water. At this stage, the battery is considered completely discharged and cannot deliver usable energy until it is recharged.
Dangers of Battery Acid
While essential for battery function, electrolyte is also very dangerous. Because the pH of battery electrolyte is below 1, it can cause:
- Severe skin burns if touched.
- Eye damage if splashed.
- Corrosion of metals and damage to car parts if leaked.
- Release of harmful gases when overcharged.
This is why manufacturers emphasize careful handling of a battery.
How to Test the Electrolyte in a Lead-Acid Battery
Checking the liquid inside a battery helps ensure it is functioning properly. A simple way to do this is with a hydrometer, which measures the specific gravity of the electrolyte.
- Using a Hydrometer:
Draw a small amount of the liquid from each cell using the hydrometer. The floating scale will show the density of the solution. - Specific Gravity Range:
- Fully charged: 1.265–1.299
- 75% charged: 1.225–1.244
- 50% charged: 1.190–1.210
- Discharged: below 1.180
- What It Indicates:
Higher readings mean the battery is charged, while lower readings indicate partial or complete discharge. - Signs of Weak or Degraded Electrolyte:
- Consistently low readings even after charging.
- Uneven readings between cells, suggesting a failing plate.
- Cloudy or contaminated liquid, reducing battery efficiency.
Regular checks of the electrolyte’s specific gravity can extend battery life and prevent unexpected failures.
Read detaile article on: How to Measure Specific Gravity of Battery?
Safety Precautions When Handling Battery Acid
If you ever need to handle, refill, or clean around a battery, follow these safety guidelines:
- Wear gloves, goggles, and protective clothing.
- Work in a well-ventilated area.
- Keep baking soda nearby to neutralize spills.
- Avoid open flames near batteries (acid + hydrogen gas = explosion risk).
- Never add tap water — always use distilled water to maintain electrolyte quality.
Battery Acid vs Other Battery Types
Not all batteries contain a liquid acid. For example:
- Lithium-ion batteries: Use lithium salts in organic solvents
- Nickel-cadmium (NiCd) batteries: Use potassium hydroxide solution
- Alkaline batteries: Use potassium hydroxide as an electrolyte
However, the batteries with acid (lead-acid type), which contains a sulfuric acid electrolyte, is still widely used in cars, UPS systems, and renewable energy storage because it is reliable and affordable.
Comparison Table: Lead-Acid vs Other Batteries
| Feature | Lead-Acid | Lithium-ion | NiCd | Alkaline |
| Electrolyte | Sulfuric acid | Lithium salts | KOH | KOH |
| Rechargeable | Yes | Yes | Yes | No |
| Safety | Corrosive | Flammable | Toxic | Safe |
| Applications | Cars, UPS, Solar | Phones, EVs | Electronics | Toys, remotes |
Quick Facts Recap: Battery Acid
- Name: Sulfuric Acid
- Chemical Formula: H₂SO₄
- Used in Car Batteries: Yes, in lead-acid batteries
- pH Level: Around 0.8 – 1.0
- Role: Acts as the electrolyte, allowing electricity to flow between the plates
Conclusion
Battery acid, also known as sulfuric acid (H₂SO₄), is the vital electrolyte that powers every battery , especially car batteries. Its extremely low acid pH makes it both powerful and hazardous. Knowing the name of battery acid, the type of acid used in car batteries, and its role helps us understand why it is important for storing energy.
While it enables millions of cars and machines to run daily, safety must always come first when dealing with it.
Related Articles: