Lead acid batteries are among the oldest and most widely used rechargeable energy storage systems. They power vehicles, UPS systems, renewable energy storage, and industrial equipment. Despite the rise of lithium-ion technology, the lead acid battery remains popular due to its reliability, low cost, and ability to deliver high surge currents.
What is a Lead Acid Battery?
A lead acid battery is a rechargeable electrochemical device that stores energy in chemical form and converts it into electrical energy when needed. It uses lead dioxide (PbO₂) as the positive active material, sponge lead (Pb) as the negative active material, and dilute sulfuric acid (H₂SO₄) as the electrolyte.
- Type: Secondary (rechargeable) battery
- Nominal cell voltage: 2V per cell
- Typical battery: 12V (6 cells in series)
Example: The standard car battery is a 12V lead acid battery designed to deliver a large burst of current to start the engine.
Construction of Lead Acid Battery
The construction is simple yet robust. The main parts include:
- Container – Made of polypropylene or hard rubber to resist acid corrosion.
- Plates –
- Positive plates: Lead dioxide (PbO₂)
- Negative plates: Sponge lead (Pb)
These are arranged in groups to increase capacity.
- Separators – Microporous PVC or glass fiber mats that prevent short circuits while allowing ionic movement.
- Electrolyte – Dilute sulfuric acid (specific gravity ~1.28 at full charge).
- Cell connectors – Lead links connecting multiple plates and cells.
- Vent plugs – Allow gases to escape while minimizing acid spillage.
Lead Acid Battery Diagram
Here is a simplified lead acid battery diagram:
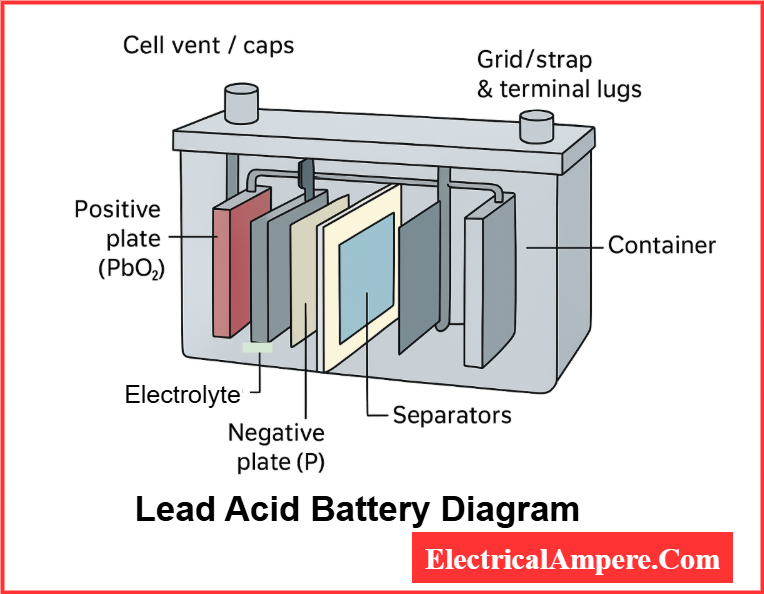
In real batteries, multiple plates are arranged in parallel to increase current capacity.
Working of Lead Acid Battery
The working principle is based on redox reactions between lead dioxide, sponge lead, and sulfuric acid.
During Discharge
- Positive plate (PbO₂) reacts with H₂SO₄ → forms PbSO₄
- Negative plate (Pb) reacts with H₂SO₄ → forms PbSO₄
- Sulfuric acid concentration decreases → water increases
Overall Discharge Reaction:
PbO2+Pb+2H2SO4→2PbSO4+2H2O
- Battery delivers current to the external circuit.
- Cell voltage drops from ~2.1V to ~1.8V.
During Charging
- External DC supply is applied.
- PbSO₄ at positive plate converts back to PbO₂.
- PbSO₄ at negative plate converts back to Pb.
- Sulfuric acid concentration increases.
Overall Charging Reaction (reverse):
2PbSO4+2H2O→PbO2+Pb+2H2SO4
Chemical Reaction of Lead Acid Battery
- Positive Plate Reaction (Discharge):
PbO2+SO24−+4H++2e−→PbSO4+2H2O - Negative Plate Reaction (Discharge):
Pb+ SO24−→PbSO4+2e−
Both plates gradually get covered with lead sulfate (PbSO₄). During charging, this reaction is reversed.
State of Charge: Voltage & Specific Gravity
For flooded lead acid batteries, the most accurate method to determine the State of Charge (SoC) is by measuring the specific gravity (SG) of the electrolyte using a hydrometer. Open-circuit voltage can also provide a quick estimate, though it is less precise.
Approximate SoC Mapping at 25 °C:
| SoC (%) | Specific Gravity (approx.) | 12 V Open-Circuit Voltage (approx.) |
| 100% | 1.255–1.275 | 12.6–12.7 V |
| 75% | 1.215–1.235 | 12.4–12.5 V |
| 50% | 1.180–1.200 | 12.2–12.3 V |
| 25% | 1.155–1.165 | 12.0–12.1 V |
| 0% | 1.110–1.130 | ≤ 11.9 V |
Note: These values are approximate. Always refer to the manufacturer’s specifications for precise guidance. Rolls Battery’s technical documentation provides similar SG vs. SoC ranges.
Practical Tip: Regular monitoring of specific gravity and voltage helps detect weak cells early, preventing unexpected failures.
Charging Lead Acid Batteries: Stages & Best Practices
Proper charging is the most important factor in maximizing the life of lead acid batteries. Understanding the stages, voltage ranges, and best practices can prevent overcharging, sulfation, and other common issues.
Charging Stages
Most lead acid batteries are charged using a three-stage process:
- Bulk Stage
- The charger delivers maximum safe current until the battery voltage reaches the absorption level.
- This stage restores most of the battery’s capacity quickly.
- Absorption Stage
- Voltage is held constant while current gradually tapers down as the battery accepts the remaining charge.
- Ensures the battery reaches full charge safely.
- Float Stage
- Maintains a lower voltage to keep the battery fully charged without causing excessive gassing or damage.
- Keeps the battery ready for use in standby applications.
Typical Voltage Ranges
Per cell / 12 V battery bank:
- Bulk / Absorption: ~2.40–2.45 V per cell → 14.4–14.7 V for 12 V battery
- Float: ~2.25 V per cell → 13.5 V for 12 V battery (range: 13.2–13.8 V)
Equalization (For Flooded Batteries)
- Occasional controlled over-voltage is applied to mix the electrolyte and prevent sulfation.
- Always check the manufacturer’s guidance for voltage and duration.
Temperature Compensation
- Charging voltage must be adjusted according to temperature:
- Lower voltage at high temperatures
- Higher voltage at low temperatures
- Many modern chargers include automatic temperature compensation to optimize charging safely.
Note: Exact voltage setpoints vary by battery type, manufacturer, and ambient temperature. Reliable references include Battery University and Rolls Battery Technical Support.
Self-Discharge and Storage of Lead-acid Batteries
Lead acid batteries naturally lose some charge over time, even when not in use. At moderate temperatures, well-maintained flooded or AGM batteries typically self-discharge around 3–5% per month. This rate increases in warmer conditions.
Storage Tips:
- Keep the battery fully charged before storing.
- Store in a cool, dry location to slow self-discharge.
- If possible, maintain a float charge to keep the battery ready.
- For offline storage, recharge every few months to prevent sulfation and capacity loss.
Proper storage significantly extends battery life and ensures reliability when the battery is needed.
Types of Lead Acid Batteries
Lead acid batteries come in several designs, optimized for different applications:
- Flooded Lead Acid Battery (FLA)
- Traditional design with liquid electrolyte.
- Requires regular maintenance and topping up of distilled water.
- Sealed Lead Acid Battery (SLA)
- Maintenance-free design with sealed housing.
- Common in UPS, emergency lighting, and alarm systems.
- Valve Regulated Lead Acid (VRLA) Battery
- Includes Absorbent Glass Mat (AGM) and Gel batteries.
- Electrolyte is immobilized, making them spill-proof and vibration-resistant.
- Deep Cycle Lead Acid Battery
- Designed for sustained energy output over long periods.
- Commonly used in solar power storage and electric vehicles.
- Starting, Lighting, and Ignition (SLI) Battery
- Provides high current for a short duration.
- Widely used in automobiles for engine starting.
Key Performance Terms in Lead Acid Batteries
Understanding these terms helps you interpret battery specifications and performance correctly.
1. Ampere-Hour (Ah)
- Indicates the battery’s energy storage capacity at a specified discharge rate.
- Example: A 100 Ah battery rated at C20 can supply 5 A continuously for 20 hours (5 A × 20 h = 100 Ah).
2. C-Rate
- Defines the discharge current relative to battery capacity.
- Common notations: C/20, C/10, 1C, etc.
- A higher C-rate means the battery is discharged faster, which affects available capacity.
3. Peukert’s Effect
- The usable capacity decreases as discharge current increases.
- In simple terms, faster drains provide fewer effective Ah than slow, steady discharges.
- Manufacturers usually provide capacity curves to account for this effect.
4. Depth of Discharge (DoD)
- Represents the percentage of battery capacity used.
- Example: DoD 50% means half the rated capacity has been used.
- Deeper discharges shorten the overall cycle life of the battery.
5. State of Charge (SoC)
- Indicates how “full” the battery is.
- Measured via specific gravity (flooded batteries) or open-circuit voltage.
- Helps plan charging and avoid over- or under-discharge.
Maintenance and Battery Health Checks
Proper maintenance ensures longer life and reliable performance for lead acid batteries. The procedures vary depending on the battery type.
For Flooded (Serviceable) Batteries:
- Check electrolyte levels monthly: Refill with distilled water only to avoid contamination.
- Monitor cell health: Use a hydrometer to measure the specific gravity of each cell. This indicates charge status and helps detect weak or failing cells.
- Clean terminals: Remove corrosion from battery terminals and clamps, and apply anti-corrosion grease to protect connections.
For Sealed Batteries (VRLA, AGM, Gel):
- No water topping is needed.
- Inspect externally for damage or leaks.
- Monitor voltage and charge behavior to ensure proper operation.
Useful Battery Tests:
- Open-circuit voltage: After letting the battery rest for 6–12 hours, measure voltage to estimate the State of Charge (SoC).
- Hydrometer reading (for flooded batteries): Provides cell-by-cell status.
- Load test or conductance test: Evaluates the battery’s ability to deliver cranking current or maintain reserve capacity.
Tip: Regular checks can prevent premature failure and help detect early signs of sulfation, overcharging, or weak cells.
Troubleshooting Quick Reference
| Symptom | Likely Cause | Quick Fix |
| Battery won’t crank but lights dim | Low state of charge / sulfation / bad cell | Fully charge battery, perform a load test, replace if a cell is faulty |
| Rapid fluid loss & excessive gassing | Overcharging or faulty voltage regulator | Check charger/alternator voltage and ensure proper ventilation |
| One cell shows low specific gravity (SG) | Cell fault or electrolyte stratification | Perform equalization charge (flooded batteries); replace cell if SG remains low |
| Battery won’t accept charge | Severe sulfation or damaged plates | Try controlled equalization; replace battery if no improvement |
Applications of Lead Acid Batteries
Lead acid batteries are versatile and widely used across many sectors due to their reliability, cost-effectiveness, and ability to deliver high surge currents. Some key applications include:
- Automobiles (SLI Batteries):
- Used for starting, lighting, and ignition.
- Typical automotive SLI batteries are 40–100 Ah, delivering high cranking currents for a few seconds.
- The vehicle alternator recharges the battery during driving.
- Power Backup Systems:
- Includes UPS systems, inverters, and emergency lighting.
- Sealed VRLA batteries are float-charged and ready to supply immediate power during outages.
- Renewable Energy Storage:
- Used in off-grid solar or wind systems.
- 12V or 48V banks of deep-cycle flooded or AGM batteries are sized based on daily energy consumption (Wh) and required autonomy days.
- Telecommunications:
- Provides backup power for telecom towers and network equipment.
- Industrial Use:
- Includes forklifts, mining equipment, and electric vehicles.
- Deep-cycle traction batteries are designed for frequent charge/discharge cycles and are often installed in ventilated battery rooms.
- Power Grids:
- Used for load leveling and energy storage in microgrids or local energy systems.
Lead Acid Battery Sizing Example (Step-by-Step)
Properly sizing a lead acid battery bank ensures enough runtime and longer battery life. Let’s go through a practical example.
Scenario:
You want a 12 V battery bank to power a 100 W load for 8 hours, while limiting Depth of Discharge (DoD) to 50%.
Step 1: Calculate Energy Requirement
Energy (Wh)=Power (W)×Time (h)
=100 W×8 h=800 Wh
Step 2: Convert Watt-hours to Ampere-hours (Ah)
Ah=Wh/Voltage (V)
Ah=800÷12≈66.7 Ah
Step 3: Adjust for Depth of Discharge (DoD)
To preserve battery life, we only want to use 50% of the total capacity: Required bank capacity=67/0.5=134 Ah
Step 4: Include Margin for Inefficiencies & Aging
Add ~15% extra capacity to account for charging inefficiencies and aging:
134×1.15≈154 Ah
Step 5: Select Battery Configuration
- Option 1: Single 12 V, 160 Ah battery
- Option 2: Two 12 V, 80 Ah batteries in parallel (depends on available space and layout)
By combining energy requirements, voltage, Depth of Discharge, and efficiency margin, you can accurately size a lead acid battery for solar setups, UPS systems, or other backup power applications.
Advantages of Lead Acid Batteries
- Low cost compared to other rechargeable batteries.
- High surge current capability → ideal for starting engines.
- Robust and reliable with simple charging methods.
- Recyclable → over 95% of lead can be reused.
Limitations of Lead Acid Batteries
- Lower energy density compared to lithium-ion.
- Heavy and bulky → less portable.
- Requires maintenance (for flooded type).
- Shorter cycle life (500–800 cycles).
Sulfation in Battery: A Key Consideration
Sulfation is a common long-term issue in lead acid batteries, occurring when lead sulfate (PbSO₄) crystals form and harden on the battery plates. This reduces capacity and, if left unaddressed, can permanently damage the battery.
Prevention tips:
- Keep the battery charged and avoid long-term discharge.
- Use chargers suited to your battery type.
- For flooded batteries, controlled equalization charges help maintain cell health.
For a detailed guide on sulfation, its causes, and recovery methods, see our full article: Battery Sulfation Explained.
Factors Affecting Lead-Acid Battery Life (Quick Overview)
The lifespan of a lead-acid battery depends on several conditions such as depth of discharge, charging method, temperature, and maintenance practices. Overcharging, deep discharges, or poor storage can significantly reduce battery life, while proper care ensures better performance.
Read the complete guide here: Factors Affecting Lead Acid Battery Life
Real-World Examples
- Car Starter Battery (12V 60Ah): Provides ~600A cranking current for engine start.
- UPS Battery (12V 100Ah): Runs home appliances during power cuts.
- Solar Battery Bank: Multiple 2V/6V lead acid cells connected for renewable storage.
Safety Precautions
Working with lead–acid batteries requires careful handling because they can pose risks if used incorrectly.
- Gas release during charging: When the battery is being charged, it can release hydrogen gas. In poorly ventilated spaces, this gas may accumulate and create an explosion hazard. Always ensure good airflow during charging.
- Corrosive electrolyte: The sulfuric acid inside the battery is highly corrosive. Direct contact can damage skin and eyes. Wearing gloves and protective eyewear is strongly recommended, and any accidental spill should be neutralized with baking soda or another suitable neutralizing agent.
- Electrical hazards: Short-circuiting the terminals can result in high currents, sparks, and even fire. Avoid placing tools or metal objects across the terminals.
Environmental Responsibility and Recycling
Lead–acid batteries are among the most successfully recycled consumer products. In many regions, more than 95% of these batteries are collected and processed at the end of their life. This high recycling rate makes them a model for sustainable resource recovery.
The recycling process allows recovery of:
- Lead – reused in new batteries.
- Plastic casings – reprocessed into new battery housings.
- Electrolyte – neutralized and repurposed.
Improper disposal, such as throwing batteries in household waste, can contaminate soil and water with lead and acid. Always return used batteries to authorized collection points or recycling centers to ensure safe handling and resource conservation.
Quick Summary – Lead Acid Battery
A lead acid battery is a rechargeable battery that stores energy using lead (Pb) and lead dioxide (PbO₂) plates submerged in sulfuric acid (H₂SO₄). During discharge, chemical reactions convert chemical energy into electrical energy through the following main reaction:
Discharge reaction:
PbO₂ + Pb + 2H₂SO₄ → 2PbSO₄ + 2H₂O
Charge reaction (reverse):
2PbSO₄ + 2H₂O → PbO₂ + Pb + 2H₂SO₄
- Type: Secondary/rechargeable battery
- Applications: Automobiles, UPS, inverters, solar power storage, grid storage
- Key Feature: Low cost, high surge current, reliable backup power
Conclusion
The lead acid battery remains one of the most dependable and cost-effective energy storage devices. By understanding its working, diagram, and chemical reactions, users can appreciate why it still dominates applications requiring reliability and high power output. While lithium-ion is growing in popularity, lead storage batteries will continue to play a critical role in automotive, industrial, and backup systems worldwide.
Related Articles:
- What is a Sulfated Battery and How to Prevent It
- Buckling of Lead Acid Battery – Causes, Effects & Prevention
- Rechargeable and Non-Rechargeable Batteries
- What Is Battery Acid? Sulfuric Acid Facts
- How to Measure Specific Gravity of Battery?
- Difference Between AA and AAA Battery
- Factors Affecting Lead Acid Battery Life and Performance