Discover the key differences between charge and mass, including their definitions, units, properties, and roles in physics. Learn how charge and mass behave in electric and magnetic fields, and why they are fundamental yet distinct physical quantities.
Charge and mass are two fundamental properties of matter. However, they differ significantly in nature and behavior. The main distinction lies in their characteristics: charge has a dual nature, meaning it can be positive or negative, while mass is always positive and does not exhibit such polarity.
Charge is associated with electric interactions, whereas mass is responsible for gravitational interactions.
What is Charge?
Charge is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. It is denoted by q and can be positive (protons) or negative (electrons).
Key Properties of Electric Charge:
- Additivity: Total charge is the sum of individual charges in a system.
- Conservation: Electric charge is conserved; it cannot be created or destroyed, only transferred.
- Quantization: Charge exists in discrete amounts. It is always a multiple of the elementary charge (e), where:
q = ne,
n is an integer, and
e = 1.6 × 10⁻¹⁹ C (magnitude of charge on an electron or proton).
Important Note: Like charges repel, and unlike charges attract. A point charge refers to a charged object whose size is negligible compared to the distance between it and other charges.
What is Mass?
Mass is a fundamental property of matter that quantifies the amount of matter in a body. It is represented by m and is always positive.
Key Features of Mass:
- Inertia: Mass is a measure of a body’s resistance to acceleration.
- Independence: Mass does not change with location or gravitational field.
- Relativity: Mass increases with speed as per Einstein’s theory of relativity:
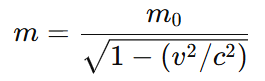
where:
m₀is rest mass,vis the velocity of the object,cis the speed of light (3 × 10⁸ m/s).
Key Differences Between Charge and Mass
- Charge can be positive or negative, while mass is always positive.
- Charge is quantized, but mass is continuous and non-quantized.
- A body cannot have charge without mass, but it can have mass without charge.
- Transfer of charge causes a minute change in mass, whereas transferring mass does not affect charge.
- Charge is invariant with speed, but mass increases with speed.
- Charge is a derived physical quantity, while mass is a fundamental one.
- Charge can cause attractive or repulsive forces, while mass only causes attractive gravitational forces.
Similarities Between Charge and Mass
- Both are intrinsic properties of matter.
- Both are scalar quantities (have magnitude, but no direction).
- Both can be added algebraically.
Charge vs. Mass: Comparison Chart
| Property | Charge | Mass |
|---|---|---|
| Definition | A property of matter causing it to experience a force in an electromagnetic field. | A measure of the amount of matter in an object. |
| Symbol | q | m |
| SI Unit | Coulomb (C) | Kilogram (kg) |
| Nature | Quantized (exists in discrete amounts) | Continuous (not quantized) |
| Types | Positive and Negative | Always Positive |
| Field Produced | Electric Field | Gravitational Field |
| Force Interaction | Can be Attractive or Repulsive | Always Attractive |
| Conservation Law | Conserved (cannot be created or destroyed) | Not strictly conserved (mass can convert to energy) |
| Dependence on Speed | Independent of speed | Increases with speed (relativistic mass) |
| Existence Dependency | Cannot exist without mass | Can exist without charge |
Summary: Key Differences Between Charge and Mass
1. Nature: Charge is a property of matter that can be positive, negative, or neutral. In contrast, mass is always positive and represents the quantity of matter present in an object. Unlike charge, there is no concept of negative mass in classical physics.
2. Type of Interaction: Charge is responsible for electromagnetic interactions. It determines how particles behave in electric and magnetic fields. On the other hand, mass is associated with gravitational interaction and plays a crucial role in determining the gravitational force between objects.
3. Unit of Measurement: The SI unit of electric charge is the Coulomb (C), whereas the SI unit of mass is the kilogram (kg). These units reflect the distinct physical quantities they represent.
4. Possibility of Transfer: Charge can be transferred from one object to another, especially in processes like friction, conduction, or induction. Mass, however, is not transferable between objects in the same way—mass remains constant unless material is physically added or removed.
5. Quantization: Charge is quantized, meaning it exists in discrete amounts and is typically a multiple of the elementary charge (±1.6 × 10⁻¹⁹ C). Mass, on the other hand, is not quantized in classical physics and can take any positive real value.
6. Conservation Law: Both charge and mass are conserved quantities in physics. Total electric charge remains constant in an isolated system. Similarly, total mass is conserved in classical mechanics, though in relativistic physics, mass can be converted to energy (as per E = mc²).
7. Dependence on External Fields: Charge responds to electric and magnetic fields and can move or experience force due to these fields. Mass is affected only by gravitational fields and contributes to gravitational attraction.
8. Effect on Inertia: Mass determines an object’s inertia, or its resistance to changes in motion. Charge has no role in inertia—it doesn’t affect how much an object resists acceleration.
9. Fundamental Property: Charge is a fundamental property related to particles like electrons and protons. Likewise, mass is a fundamental property that all physical substances possess, whether charged or neutral.
10. Polarity: Charge has polarity—it can be positive or negative. This dual nature enables phenomena like attraction and repulsion. Mass lacks polarity; it only causes attraction through gravity and has no repelling behavior.
Conclusion
Both proton and electron are fundamental particles that illustrate the concept of charge and mass:
- Proton:
- Charge = +1.6 × 10⁻¹⁹ C
- Mass = 1.67 × 10⁻²⁷ kg
- Electron:
- Charge = -1.6 × 10⁻¹⁹ C
- Mass = 9.11 × 10⁻³¹ kg
Even though both particles have mass and charge, their charge types and mass values are very different. This comparison shows how charge and mass are different but still connected in the physical world.
FAQs on Charge and Mass
1. What is the charge and mass of a proton?
- Charge of proton (q): +1.602×10−19 Coulombs
- Mass of proton (m): 1.673×10−27 kilograms
2. What is the charge to mass ratio of a proton?
The charge-to-mass ratio of a proton is:
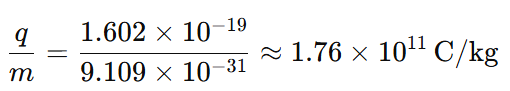
3. What is the charge to mass ratio of an electron?
For an electron:
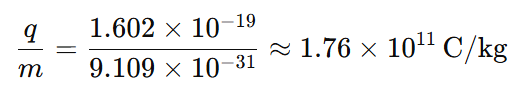
4. What is the charge and mass formula?
There’s no single formula combining charge and mass, but in electromagnetic fields, the charge-to-mass ratio is q/m=used in motion analysis under electric and magnetic fields
5. What is the charge of an electron?
The charge of an electron is: q=−1.602×10−19 Coulomb
(Note: It’s negative, opposite to proton)
6. What is the formula with charge and mass?
One commonly used formula is force under electric field:
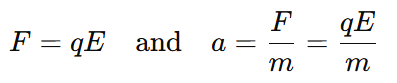
This shows how acceleration depends on both charge and mass.
7. What is the relationship between charge and mass?
There is no direct physical relationship between charge and mass; they are independent properties. However, their ratio (q/m) is critical in analyzing particle behavior in fields (e.g., in cyclotrons or mass spectrometers).
8. Does mass increase with charge?
No, mass does not increase with charge. Both are intrinsic properties of particles and are not interdependent.
Read Next: